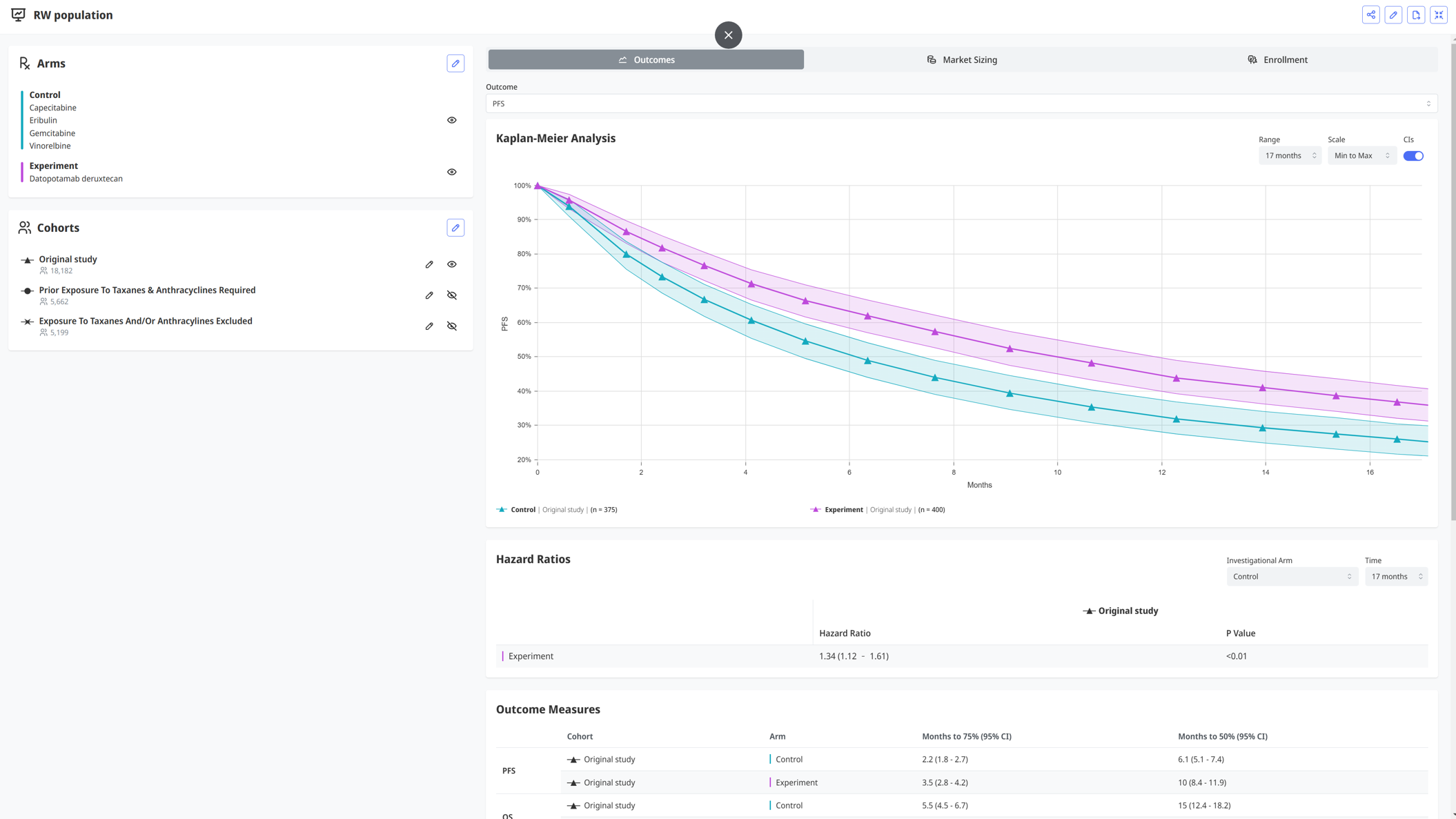
Protocol Design & Optimization
Our platform allows the trial design team to modify any protocol parameter, and assess its clinical, operational, and commercial impact. For instance, you can optimize the I/E criteria to maximize the clinical effect size, without compromising on feasibility and commercial impact. Additionally, you can assess different drug combinations to maximize efficacy, while minimizing safety concerns. The platform is highly adaptable and allows for granular modifications on the treatment arms, comparators, outcomes, endpoints, inclusion/exclusion criteria, analysis methods, and more.
Additional Modules
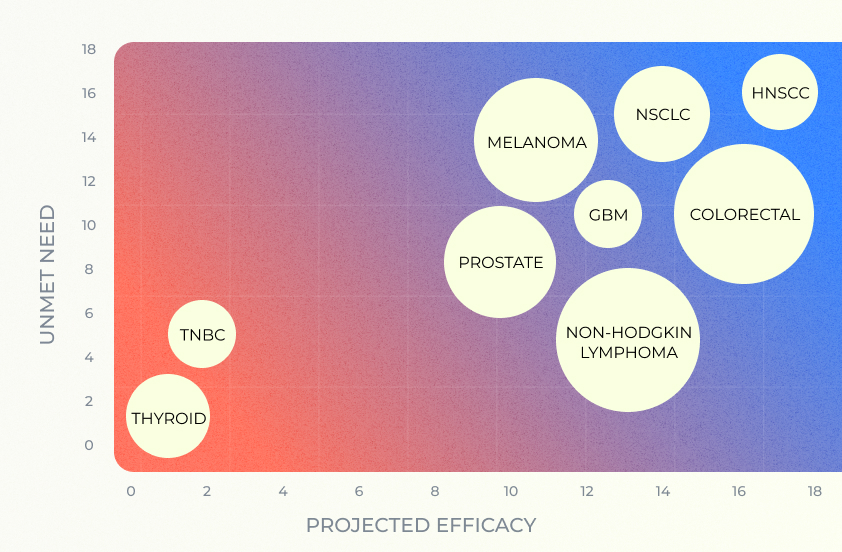
Indication Selection
Our AI-based indication selection module predicts the efficacy and safety of drugs across various medical conditions, drawing on the immense variability within, but also across various diseases. By presenting the results in an intuitive and clinically relevant manner, this tool enables precise and efficient decision-making in early clinical development.
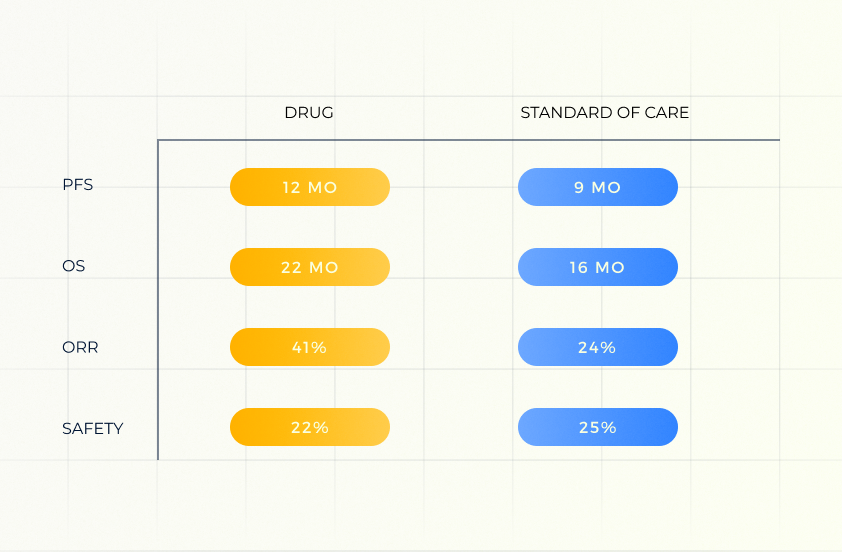
TPP
Our AI-based Target Product Profile (TPP) module predicts key TPP parameters, focusing on primary endpoints and safety. This tool enhances strategic decision-making by providing reliable perspective on the therapeutic potential and safety profile of the asset.
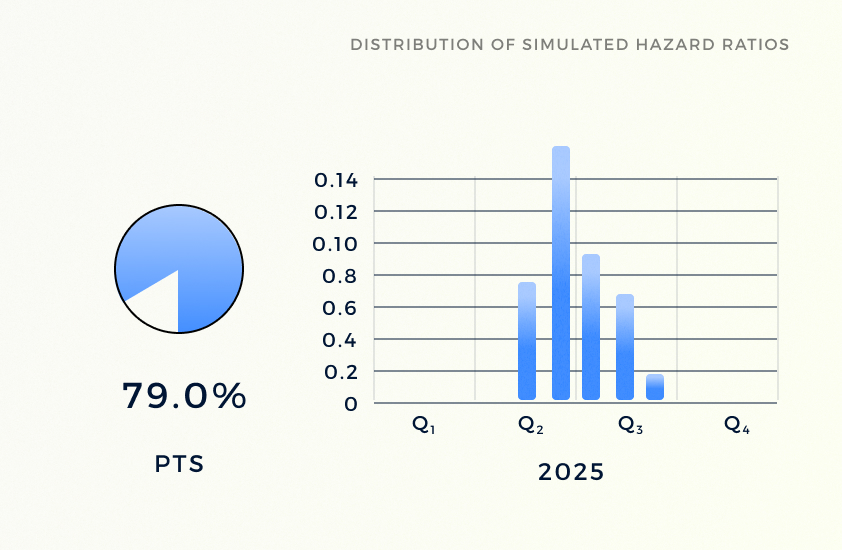
PTS
By integrating rigorous efficacy and safety simulation with real-world evidence, QuantHealth's PTS (probability of technical success) module aids in optimizing clinical trial investments, minimizing risk, and enhancing decision-making processes in drug development.
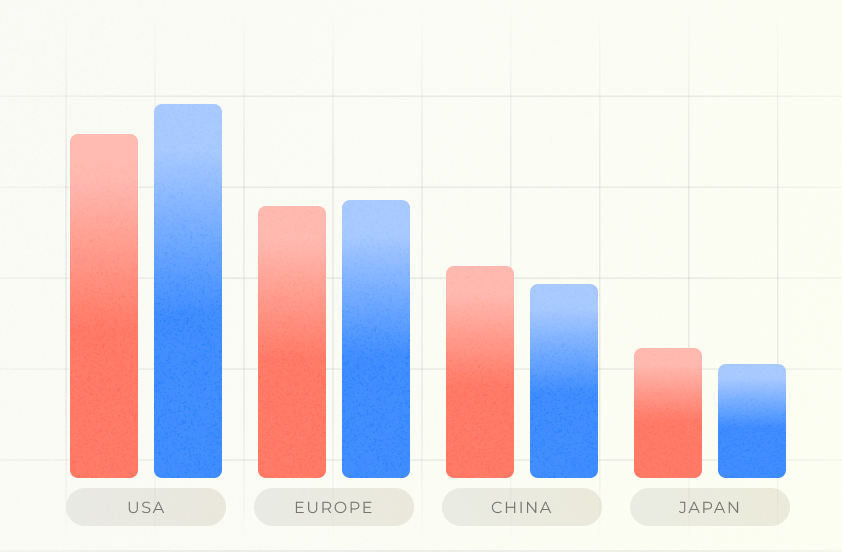
Market Forecasting
Predict the expected number of patients in various geographical markets based on a specific cohort definition. This module allows to compare the global patient population across different cohorts, enabling more informed and strategic planning.
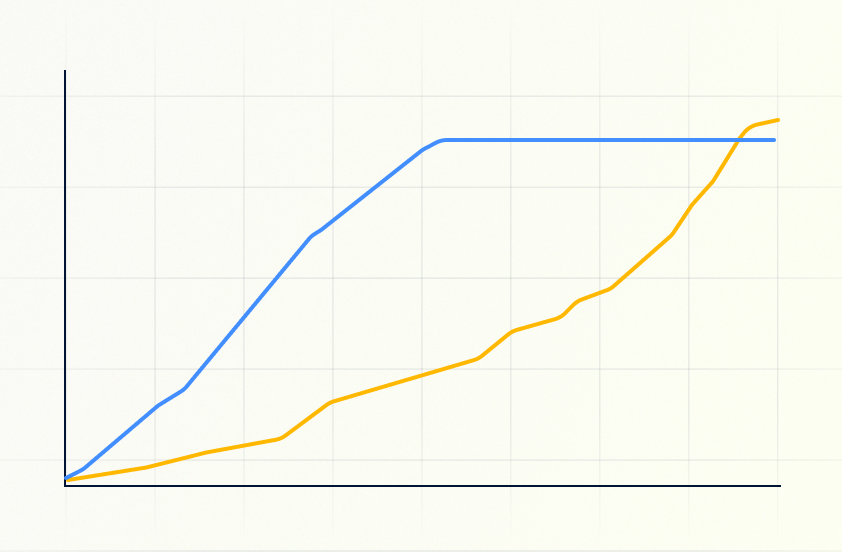
Enrollment Prediction
The patient recruitment module predicts site-level and global trial enrollment over time by examining the cohort itself as well as hundreds of protocol parameters such as protocol complexity, site burden, estimated site performance, investigator, and more. This module supports site selection, feasibility analysis, and provides an essential operational perspective to any trial design team.