Introduction/Background
The customer team was challenged with creating a successful protocol trial design for upcoming phase 2b and future phase 3 to achieve target end point for novel therapeutic.
QuantHealth's ability to integrate population PK-PD models and simulate the impact of such novel treatments using AI models trained on very large clinical datasets, encouraged the team to explore this technology to run thousands of trials in silico to help define the optimum protocol design.
The customer chose to partner with QuantHealth over internal capabilities and other vendors due to:
Depth of data - A GenAI platform harnessing and combining data from >350M patients and >100,000 drug entities
Platform - An advanced analytics module that the data science team could use with minimal training
Customer support - A consulting and support team that provided the necessary hand-holding to enable the respiratory disease team
Methodology
A real-world data AI driven simulation was developed predicting the asset's impact on disease progression. The sponsor's population PK-PD models were reproduced and integrated with the disease progression model. Simulations were conducted predicting changes in biomarker and in clinical outcome by dosing regimen and patient subgroups and applied to real-world data. Simulations were conducted for competitor as well to provide insight on competitive advantages.
Results
Simulations supported the selection of a dosing regimen which ensured high probability of success while minimizing safety risk. Exploring simulations across subgroups allowed to demonstrate that patient weight had minimal impact on the dosing required to achieve outcome.
Competitive comparisons highlighted features differentiating Sponsors' asset from competitor with similar MoA and their expected impact on clinical outcomes – these included longer half-life leading to increased potential for a more convenient regimen and lower variability in effects across time.
Population PK-PD Simulation
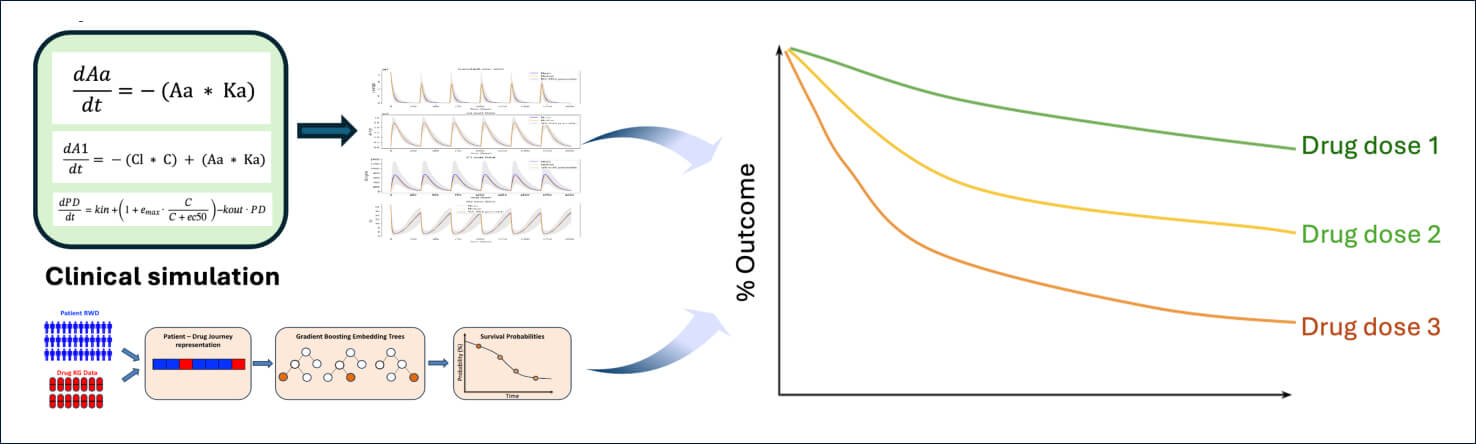
Net Benefits
Enabled sponsor to move from first-in-human to proof-of-concept phase 2b, including:
Selection of a dosing regimen across subgroups and ensuring high probability of success & minimizing safety risk.
Dose clinical response modeling established optimal dose range and allowed reduction of arms from 4 to 2, and reduced time to last patient first dose (LPFD).
Confirmed a uniform dosing approach
Incremental Financial Return
| Insight | Operational Gain | Financial Return |
|---|---|---|
| Dose optimization reducing number of arms in phase 2b trial from four to two. | 140 fewer patients | $8.88M(~$63K/patient) |
| Time to LPFD reduced by 140/15 patients per month = 9.3 months reduction in trial length. | $141M-Value of reduced time to market and increased time in peak sales |