Introduction/Background
The customer team was challenged with creating a successful protocol trial design that would achieve the target end point within 9 months with shrinking budget.
This improbable time frame encouraged the team to explore advances in artificial intelligence/GenAI technology that can run thousands of trials in silico to help define the optimum protocol design that would net the desired outcome.
The customer chose to partner with QuantHealth over internal capabilities and other vendors due to:
Depth of data - A GenAI platform harnessing and combining data from >350M patients and >100,000 drug entities
Platform - An advanced analytics module that the data science team could use with minimal training
Customer support - A consulting and support team that provided the necessary hand-holding to enable the respiratory disease team
Methodology
An initial sponsor protocol was simulated within a drug-specific model to evaluate expected primary outcome result. QuantHealth simulated >5,000 protocol variations, to determine which factors were most likely to contribute to success. Out of >30 positive protocols, customer chose the following protocol that suggested highest odds of technical success.
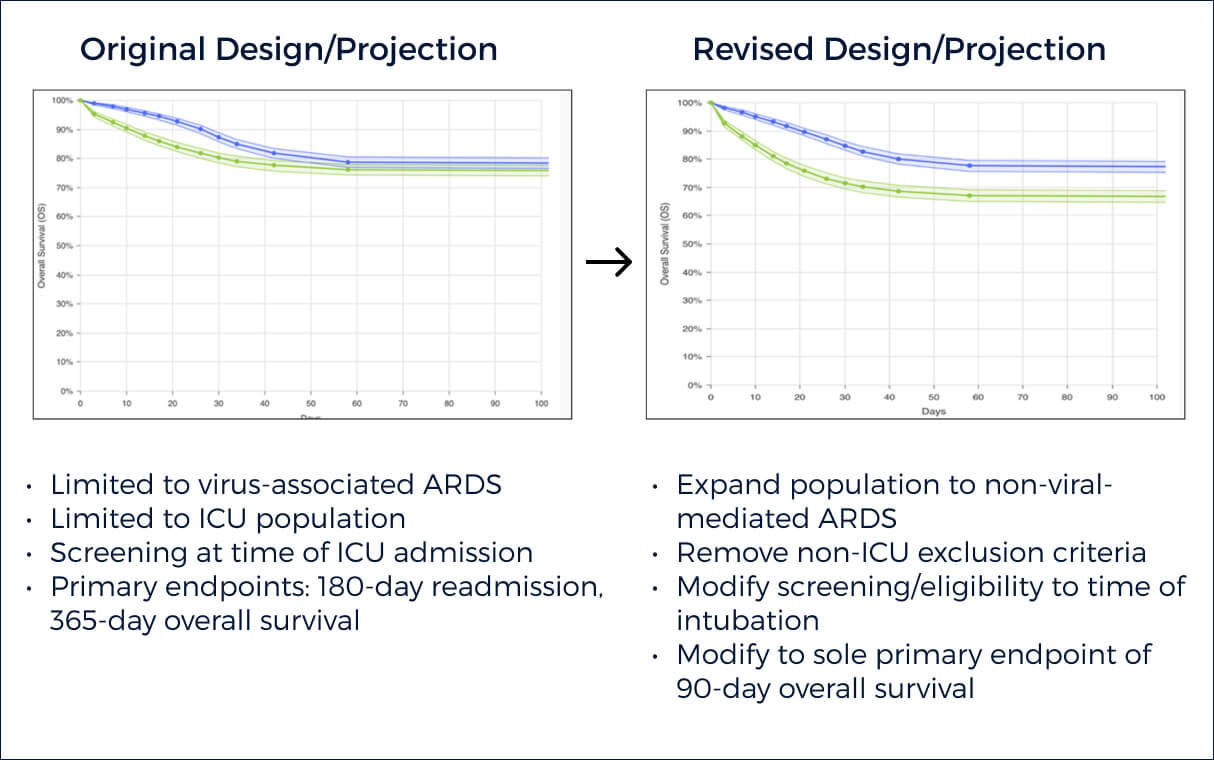
Net Benefits
| Metric | Operational Gain | Incremental Financial Return |
|---|---|---|
| Enrollment time | mo 11 | $15m in # of sites and duration of site monitoring; ~$200m in projected peak sales (due to earlier launch |
| Clinical trial subjects | subjects 251 | |
| P3IND; protocol design | FTE 1.5~ | $385,000 |